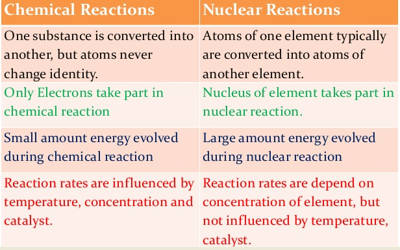
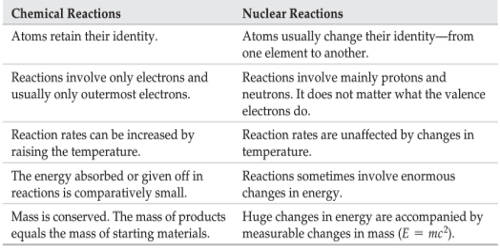
Chemical reactions take place outside the nucleus in the atoms electron cloud. The rate of reaction in a nuclear reaction is spontaneous while in the chemical reaction is non-spontaneous.
Difference Between Chemical Reactions And Nuclear Reactions Qs Study
While a chemical reaction requires or gives off a relatively small amount of energy through the breaking and forming of atomic bonds a nuclear reaction gives off a large amount of energy through the splitting fission or fusing fision of atomic neclei.

. Difference Between Chemical Reactions and Nuclear Reactions. In nuclear reactions the. These are low energy transactions.
Thus we can conclude that nuclear reactions are different from chemical reactions as follows. A chemical reaction is either reversible or irreversible while the nuclear reaction is irreversible. 1 Nuclear reactions involve a change in an atoms nucleus usually producing a different element along with the emission of radiations like αβ and γ etc rays.
Different isotopes of an element normally behave similarly in chemical reactions as their extra-nuclear electronic configurations are same. These changes in energy levels allow for the formation of new bonds with other atoms and molecules. Nuclear reaction happens only inside the nucleus.
Chemical reaction can be influenced by pressure or temperature. Rate of a chemical reaction is largely affected by temperature and pressure. Chemical reactions result only in the transfer of electrons.
The chemical reactions involve the transfer loss gain and sharing of electrons and nothing takes place in the nucleus. Lets have a look at the differences below. New molecules are formed.
While nuclear reaction takes place in the atoms nucleus the electrons in the atom are responsible for Chemical reactions. During nuclear reactions the nuclei of atoms undergo change and therefore new elements are formed as a result of such reactions. During chemical reactions elements remain the same and do not lose their identity.
Li6-D Reaction by. In nuclear reactions the identities of the elements change. But In Nuclear reactions the the nucleus in the reacting element and the produced element are not same.
Here is what you want to know about the Difference Between Nuclear and. Spread the Differences. During such reactions there is low energy change.
Nuclear reactions involve the decomposition of the nucleus and have nothing to do. The main difference between nuclear reaction and chemical reaction can be understood on the basis of how the reaction takes. Reactivity of an element towards chemical reactions depends upon the oxidation state of the element.
The main difference between chemical reaction and nuclear reaction is based on how or where the reaction takes place in the atom. What these mean and how they differ from each other are discussed below. Chemical reactions are dependent of the chemical form of the element.
Nuclear Reaction vs Chemical Reaction. Nuclear reaction is independent of such factors. Nuclear reactions are those reactions in which nuclear transformation takes place.
Chemical reaction is a process in which atoms of an element rearrange themselves to form a new substanceNuclear reaction is a process in which the structure of the nucleus of an atom is changed with the release of energy. Answer 1 of 19. The maximum number of electrons surrounding the nucleus equals the number of protons in the nucleus.
Energy changes accompanying nuclear reactions are relatively higher and larger. In ordinary chemical reactions Ra and Ra 2. Nuclear reactions involve changes in.
Chemical reactions involve changes in the energy levels of orbiting electrons. During nuclear reactions only the nucleus is affected. Six differences between nuclear reaction and chemical reaction.
All the changes taking place in the environment are due to either chemical or nuclear reactions. During chemical reactions elements do not lose their identity. For example is a chemical change.
The chemical reactions involve the transfer loss gain and sharing of electrons and nothing takes place in the nucleus. While nuclear reaction takes place in the atoms nucleus the electrons in the atom are responsible for Chemical reactions. Nuclear reactions involve the decomposition of the nucleus and have nothing to do.
Combustion reaction of methane by Jynto Robert A. Chemical reactions on the other hand involve only a rearrangement of electrons and do not involve changes in the. The chemical reactions involve the transfer loss gain and sharing of electrons and nothing takes place in the nucleus.
Whereas a chemical reaction is defined as the reaction where there occurs exchange of electrons between the combining atoms. Rohde Jacek FH CC BY-SA 30 via Commons. When chemical reactions occur elements hold their identity and.
Nuclei of atoms undergo complete change during nuclear reactions creating new elements. A chemical reaction can be reversed. Chemical reaction is a process of converting a set of substances into another set of substances.
Another difference is the amount of energy required. Chemical reactions are accompanied by relatively small energy changes. Nuclear reactions only occur among highly unstable atoms and are usually created on purpose.
The nuclear reaction occurs in highly unstable and radioactive atoms while chemical reaction can occur at any time in life. Chemical reactions are the basis of life and it occurs all aroundin us at any given time. As the name implies nuclear reactions occur in the nucleus of an atom.
In these reactions only the electrons in the outermost shell of atoms participate whereas the nuclei of atoms remain unchanged. The main difference between these phenomena is in how the reaction occurs at the atomic level. Chemical reactions are the most common type of atomic interaction on Earth and the one that most people remember learning.
Elements preserve their identity and the nuclei of atoms remain unchanged during chemical reactions. Primarily chemical reactions only involve the electrons of an atom while nuclear reactions will involve the nucleus of an atom. Chemical reactions involve electromagnetic force.
A chemical reaction is the process in which reactants react chemically and convert into products by chemical transformation. Chemical reaction normally occurs outside the nucleus.
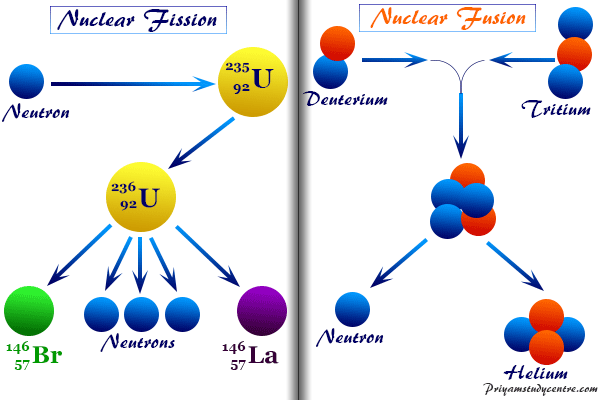
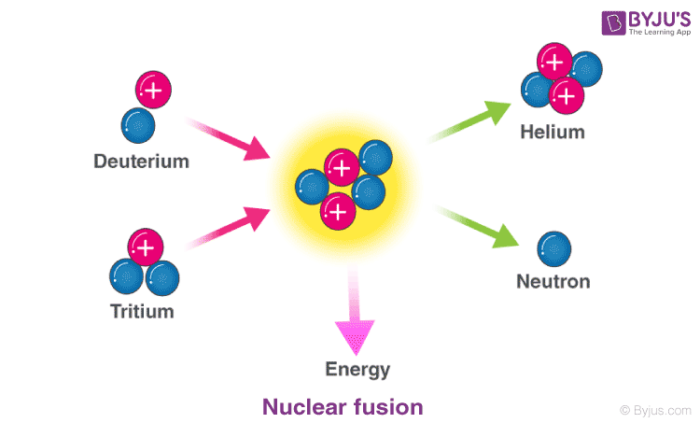
Nuclear Reaction Types Definition Examples
Difference Between Chemical Reactions And Nuclear Reactions Qs Study

Difference Between Chemical Reaction And Nuclear Reaction Brainly In
0 Comments